Interested in learning more about how REZZAYO® rezafungin could help in your hospital?
Request an appointment with a Napp representative
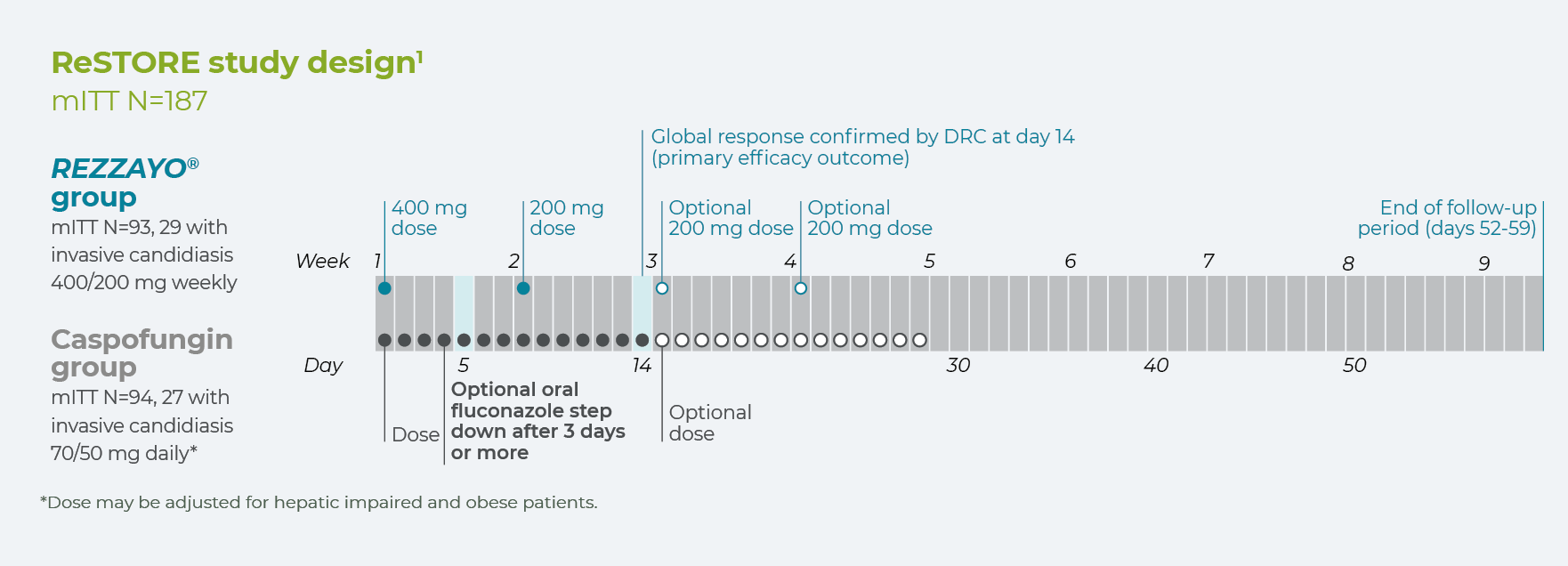
ReSTORE
ReSTORE was a prospective, double blind, randomised, non-inferiority phase III trial of once-weekly intravenous REZZAYO® vs daily caspofungin for the treatment of invasive candidiasis and/or candidaemia in adults, conducted at 66 tertiary care centres in 15 countries. 199 patients meeting inclusion criteria were 1:1 randomised. Randomisation was stratified based on diagnosis, modified APACHE II score, and absolute neutrophil count.1
Please note that REZZAYO® is indicated for the treatment of invasive candidiasis in adults.
*Dose may be adjusted for hepatic impaired and obese patients.
Learn more about the ReSTORE trial
ReSTORE and STRIVE pooled analysis
The pooled analysis, published in The Lancet Infectious Diseases, of ReSTORE (phase III) and STRIVE (phase II), was a pre-planned pooled analysis of two international, multicentre, double-blind, randomised controlled trials.3

Learn more about the pooled study design and results in this study summary
DRC, Data Review Committee; IV, intravenous; mITT, modified intent-to-treat.
- REZZAYO® (rezafungin). Summary of Product Characteristics. Napp 2024.
- Thompson GR III, et al. Lancet. 2023;401(10370):49–59.
- Thompson GR III, et al. Lancet ID. 2023; doi: 10.1016/S1473-3099(23)00551-0.
Adverse events should be reported. Reporting forms and information can be found at http://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Napp Pharmaceuticals Limited on 01223 424444 or drugsafetyUKandROI@mundipharma.com.
®: REZZAYO is a Registered Trademark of Napp Pharmaceutical Group Limited.










