A significant healthcare burden

Invasive candidiasis, an increased burden linked to advances in medical technology, is widely recognised as a major cause of morbidity and mortality.2
Candida is the most common cause of life-threatening fungal infection accounting for 70% to 90% of all invasive fungal infections.3
There are five Candida species that account for more than 90% of all invasive candidiasis diagnoses: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei.5
Learn more about the burden of invasive candidiasis
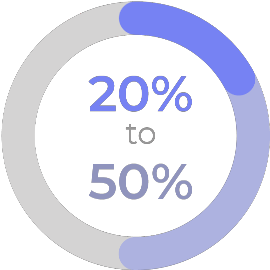
overall mortality
of invasive candidiasis.4
Candida is the most common cause of life-threatening fungal infection accounting for 70% to 90% of all invasive fungal infections.3
There are five Candida species that account for more than 90% of all IC/C diagnoses: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei.5
Learn more about the burden of invasive candidiasis
Which patients are at risk for invasive candidiasis?
The risk of invasive candidiasis is increased for critically ill and immunocompromised patients, such as those being treated for cancer or undergoing transplants.2,6
Other risk factors associated with invasive candidiasis include:2,6,7
Renal impairment
Use of mechanical ventilation
Hospital stays longer than 10–15 days
Central venous catheterisation
Sepsis
Total parenteral nutrition
Use of broad-spectrum antibiotics
Increased age
Rare Candida spp. as causative pathogens
Abdominal surgery
Discover more about the invasive candidiasis risk factors
Diagnosing invasive candidiasis: a major challenge
There are no distinct clinical signs or symptoms that are specific for invasive candidiasis.2
Invasive candidiasis should always be suspected in patients:2
- with known risk factors
- who have unexplained fever
- who are unresponsive to antibacterial treatment2
Explore the diagnosis of invasive candidiasis
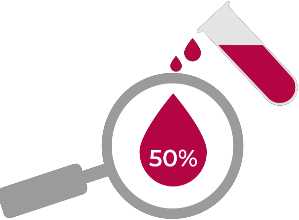
Blood cultures may miss
half of all cases.8
Explore the diagnosis of invasive candidiasis
The challenge of managing drug-drug interactions (DDIs) in invasive candidiasis
- Some antifungals used to treat invasive candidiasis are frequently linked with drug-drug interactions, including cytochrome P450 (CYP) mediated interactions with drugs such as venetoclax, idelalisib, ruxolitinib, cyclosporine, and rifampicin9-13
- Over the last decade, an increase in DDIs due to the emergence of targeted therapies for haematological malignancies has further increased treatment challenges13
- Most of these targeted therapies for haematological malignancies are metabolised by the CYP enzymes in the liver. This predisposes these drugs to potentially severe DDIs, especially when used in conjunction with triazoles that are moderate-to-strong inhibitors of CYP3A413
Learn more about the challenges of managing DDIs
16% of patients in 20 different European countries suffering from candidaemia experienced a prolonged hospital stay, which was attributed to the need for parenteral antifungal treatment (n=621 from study)7
See further information on managing Invasive candidiasis in the ICU
▼REZZAYO® (rezafungin) click for GB prescribing information or NI prescribing information
CYP, cytochrome P450; DDI, drug-drug interaction; ICU, intensive care unit.
- Brown G, et al. Med Mycology. 2012;4(165)1-9.
- Pappas PG, et al. Nat Rev Dis Primers. 2018;4:18026.
- Delaloye J, Calandra T. Virulence. 2014;5(1):161–169.
- WHO fungal priority pathogens list to guide research, development and public health action. Available from: https://www.who.int/publications-detail-redirect/9789240060241. [Last accessed March 2024].
- Antinori S, et al. Eur J Intern Med. 2016;34:21–28.
- Kullberg BJ, Arendrup MC. N Engl J Med. 2015;373(15):1445–1456.
- Hoenigl M, et al. Lancet Infect Dis. 2023;23(6):751–761.
- Clancy CJ, Nguyen MH. J Clin Microbiol. 2018;56(5):e01909-17.
- Flanagan S, et al. Microbiol Spectr. 2023;11(3):e0133923.
- Caspofungin. Summary of Product Characteristics.
- Micafungin. Summary of Product Characteristics.
- Amphotericin B. Summary of Product Characteristics.
- Brüggemann RJ, et al. Lancet Haematol. 2022;9(1):e58–e72.
Adverse events should be reported. Reporting forms and information can be found at http://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Napp Pharmaceuticals Limited on 01223 424444 or drugsafetyUKandROI@mundipharma.com.









