*No dose adjustments for patients with hepatic or renal impairment, elderly (≥65 years) or obese (body mass index ≥30) patients, and can be administered independently of the timing of haemaodialysis.1
†The need for dose adjustments is considered unlikely for medicinal products that are substrates for the CYP2C8, CYP3A4, CYP1A2, and CYP2B6 enzymes and P-gp, BCRP, OATP, OCT1, OCT2, MATE1, and MATE2 transporter proteins, when administered with rezafungin. The drug-drug interaction potential of rezafungin with a number of co-administered medicinal products has also been assessed clinically. The need for dose adjustments is considered unlikely for tacrolimus, cyclosporine, ibrutinib, mycophenolate mofetil, and venetoclax when administered with rezafungin.
Interested in learning more about how REZZAYO® could help in your hospital?
Request an appointment with a Napp representative
One IV infusion for approximately 1 hour, once weekly1
Recommended dose (supplied as a single-dose vial containing 200 mg of rezafungin):1

- The safety information on REZZAYO® treatment durations longer than 4 weeks is limited1
- REZZAYO® is supplied as a single-dose vial containing 200 mg of rezafungin1
- Treatment should be initiated by a physician experienced in the management of invasive fungal infections1
- After reconstitution and dilution, the solution should be administered by slow intravenous infusion1
- An infusion may be slowed, or paused and restarted at a lower rate if infusion-related reactions occur1
- The duration of treatment should be based upon the patient’s clinical and microbiological response. In general, antifungal therapy should continue for at least 14 days after the last positive culture1
Explore the patient support materials
REZZAYO® rezafungin dosing1
- For intravenous use only:1
- The need for dose adjustments of REZZAYO® rezafungin is considered unlikely when co-administered with other medicinal products and not required for special populations 1,2+
- No dose adjustments are required for:1
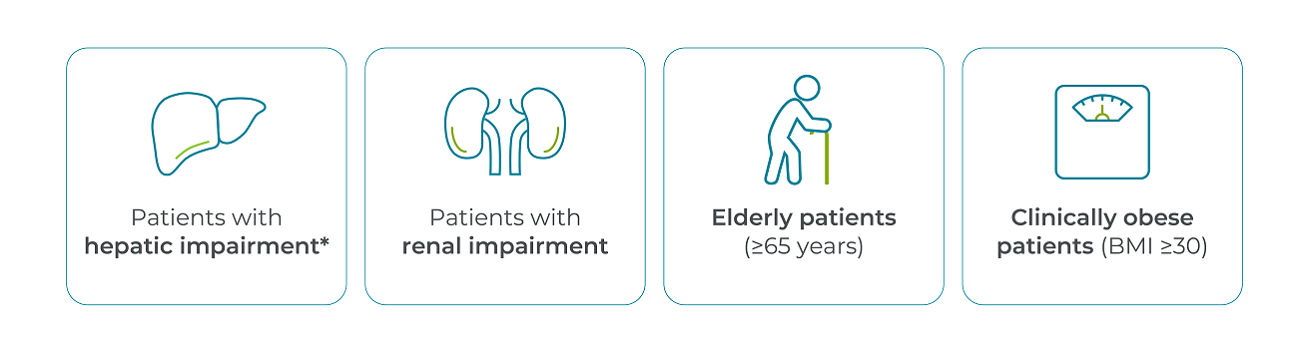
- Can be administered independently of the timing of haemodialysis1
+The need for dose adjustments is considered unlikely for medicinal products that are substrates for the CYP2C8, CYP3A4, CYP1A2, and CYP2B6 enzymes and P-gp, BCRP, OATP, OCT1, OCT2, MATE1, and MATE2 transporter proteins, when administered with rezafungin. The drug-drug interaction potential of rezafungin with a number of co-administered medicinal products has also been assessed clinically. The need for dose adjustments is considered unlikely for tacrolimus, cyclosporine, ibrutinib, mycophenolate mofetil, and venetoclax when administered with rezafungin.
Find out more about REZZAYO®: the first once-weekly echinocandin
The need for dose adjustments of rezafungin is considered unlikely1,6
REZZAYO® had no or minimal effects on the exposure of probe substrates for the following CYP enzymes/transporter proteins:
- CYP2B6 (efavirenz)
- CYP3A4 (midazolam and tacrolimus)
- CYP1A2 (caffeine); CYP2C8 (repaglinide)
- P-glycoprotein (P-gp) (digoxin and tacrolimus);
- OCT-1, OCT-2, MATE-1, and MATE-2 (metformin)
- organic anion transporting polypeptides (OATP) (pitavastatin, rosuvastatin, and repaglinide)
- breast cancer resistance protein (BCRP) (rosuvastatin).1,6
The drug-drug interaction potential of rezafungin with a number of co-administered medicinal products has also been assessed clinically. The need for dose adjustments is considered unlikely for tacrolimus, cyclosporine, ibrutinib, mycophenolate mofetil, and venetoclax when administered with rezafungin.
Find out more about the pharmacokinetics of REZZAYO®
Convenient storage
- Unopened vials: do not store above 25oC
- Keep the vial in the outer carton in order to protect from light
- REZZZAYO® does not require refrigeration before reconstitution, however, the reconstituted solution and the diluted solution for infusion should be used immediately. Refer to the REZZAYO® SmPC for full instructions for use.
The longest-acting echinocandin1–4
- REZZAYO® has distinct structural feature which confers greater stability, compared to other echinocandins, resulting in a prolonged half-life of 5–6 days, enabling once-weekly dosing1-5
- The first dose (400 mg) of REZZAYO® yields high plasma drug concentrations early in therapy and, together with subsequent 200 mg doses, provides fungicidal coverage through the treatment course7,8
- Steady state was achieved with the first loading dose1
- Area under curve/minimum inhibitory concentration (AUC/MIC) values are maintained throughout the dosing interval9+
+AUC/MIC assessed in preclinical model.
Discover the benefits of REZZAYO® mechanism of action (MOA)
AUC, area under curve; BCRP, breast cancer resistance protein; BMI, body mass index; CYP, cytochrome P450; DDI, drug–drug interaction; IV, intravenous; MATE, multi-drug and toxin extrusion protein; MIC, minimum inhibitory concentration; MOA, mechanism of action; OATP, organic anion transporting polypeptides; OCT, Organic Cation Transporter; P-gp, P-glycoprotein.
- REZZAYO® (rezafungin). Summary of Product Characteristics. Napp 2024.
- Caspofungin. Summary of Product Characteristics.
- Micafungin. Summary of Product Characteristics.
- Anidulafungin. Summary of Product Characteristics.
- Krishnan BR, et al. J Antibiotics. 2017;70:130–135.
- Flanagan S, et al. Microbiol Spectr. 2023;11(3):e0133923.
- Sandison T, et al. Antimicrob Agents Chemother. 2017;61:e01627–16.
- Data on file, Mundipharma, REF-16722.
- Lepak AJ, et al. Antimicrob Agents Chemother. 2018;62(2):e02154-17.
Adverse events should be reported. Reporting forms and information can be found at http://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Napp Pharmaceuticals Limited on 01223 424444 or drugsafetyUKandROI@mundipharma.com.
®: REZZAYO is a Registered Trademark of Napp Pharmaceutical Group Limited.










