Explore the benefits of flutiform® pMDI for children with asthma
1
flutiform® pMDI 50/5 μg improves lung function and asthma control compared to baseline, with comparable efficacy to fluticasone propionate/ salmeterol pMDI§ and demonstrated a similar safety and tolerability profile¶ vs fluticasone propionate/ salmeterol pMDI in children aged 5 to 12 years.5
2
flutiform® pMDI 50/5 μg has demonstrated superiority to fluticasone propionate pMDI† and non-inferiority to fluticasone propionate/ salmeterol pMDI‡ for change in pre-dose FEV₁ to 2 hours post-dose over 12 weeks.4
5
flutiform® pMDI 50/5 μg can be used with a spacer in patients who find it difficult to synchronise actuation and inspiration3
- GINA 2021 mentions that using pMDIs with spacers improves delivery and reduces the potential for side effects caused by ICS.6
- Learn how to use flutiform® pMDI via the instruction video and step-by-step guide.

4
flutiform® pMDI 50/5 μg has not shown effects on plasma cortisol or children's growth5
- No hypothalamic-pituitary-adrenal axis suppression was observed in a 24-week study with asthmatic children. Mean and median plasma cortisol values remained stable throughout.5
- Children’s mean height increased by 2.8 cm during the extension phase of the study.5
3
Fast-acting LABA3†
- Onset of bronchodilation within 1-3 min3†
†flutiform® pMDI inhaler is not indicated for use as a reliever.3
†LS mean difference (flutiform® pMDI versus fluticasone propionate pMDI) was 0.07L [95% CI: 0.03, 0.11L, p < 0.001 (superiority)].4
‡LS mean difference (flutiform® pMDI versus fluticasone propionate/ salmeterol pMDI) 0.00L [95% CI: −0.04, 0.04L, p < 0.001 (non-inferiority)].4
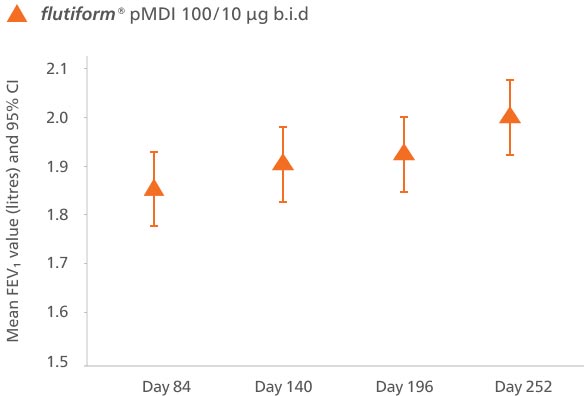
§flutiform® pMDI 100/10 μg b.i.d. shown non-inferior to fluticasone propionate/ salmeterol pMDI 100/50 μg b.i.d. for change in pre-dose FEV₁ from day 0 to day 84. During the extension phase, day 84 to day 252 (full-analysis set), flutiform® pMDI 100/10 μg b.i.d. provided sustained improvements in FEV₁ over 36 weeks, subsequently increased by 0.105 L from 1.85 L shown at day 84.5
¶flutiform® pMDI 100/10 µg b.i.d. demonstrated acceptable tolerability over 36 weeks vs fluticasone propionate/ salmeterol 100/10 µg b.i.d.5
Abbreviations: b.i.d., twice daily; CI, confidence interval; FEV₁, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; LABA, long-acting β₂ agonist; LS, least square; pMDI, pressurised metered-dose inhaler
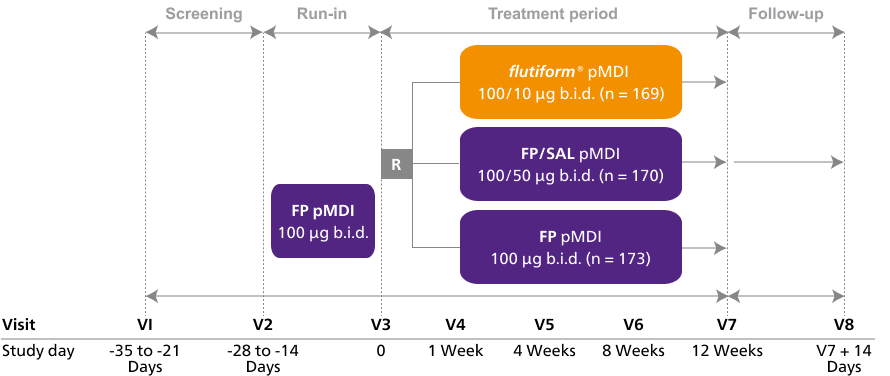
Clinical studies (children)
flutiform® pMDI 50/5μg has demonstrated efficacy in children aged 5 and above.4,5
ffLAIR4
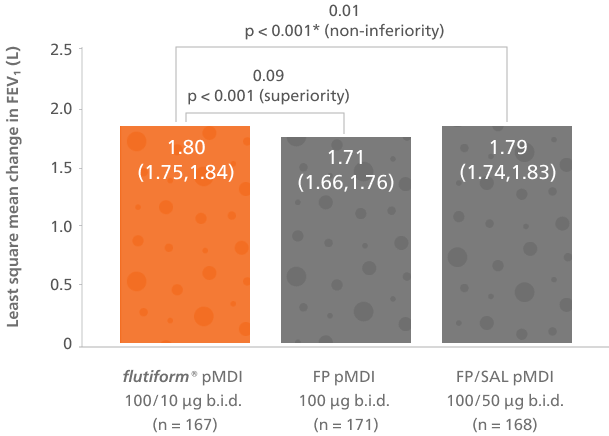
flutiform® pMDI 50/5 µg has demonstrated superiority to fluticasone propionate (FP) pMDI 50 µg and non-inferiority to fluticasone propionate/salmeterol (FP/SAL) pMDI 50/25 µg for change in FEV₁ from pre-dose at baseline to 2-hour post-dose over 12 weeks.4
ffLAIR4: A randomised parallel-group trial to further evaluate the efficacy and safety of flutiform® pMDI 50/5 µg in the paediatric population
Abbreviations: AUC, area under the curve; FEV₁, forced expiratory volume in 1 second; FP, fluticasone; CI, confidence interval; FP/SAL, fluticasone/salmeterol; pMDI, pressurised metered-dose inhaler
Key Outcomes:4
flutiform® pMDI 50/5 µg was superior to FP pMDI and non-inferior to FP/SAL pMDI in the primary endpoint and in changes in pre-dose FEV₁ from baseline over the 12-week treatment period4
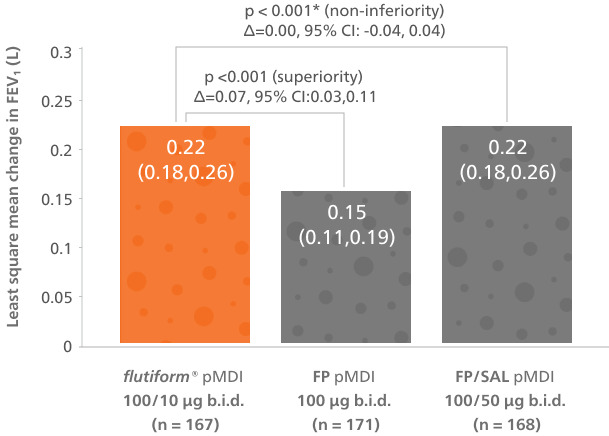
Adapted from Ploszczuk A, et al. 2018.
Least squares mean change from predose FEV₁ at baseline to 2 hours post-dose over the 12-week treatment period, full analysis population.*Based on pre-specified non-inferiority margin of -0.1L.
36-week paediatric trial study5
flutiform® pMDI 50/5 μg improved lung function and asthma control compared to baseline, with comparable efficacy to fluticasone propionate/salmeterol (FP/SAL) pMDI 50/25 μg and demonstrated similar safety and tolerability profile vs fluticasone propionate/ salmeterol (FP/SAL) pMDI 50/25 μg in children aged 5 to 12 years5
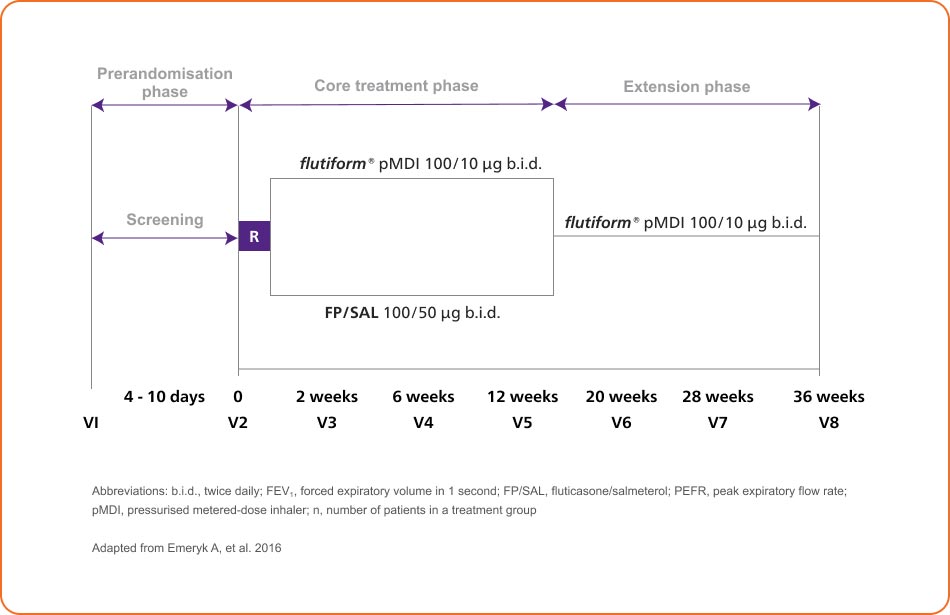
12-week open-label, randomised, controlled trial and 24-week extension to assess the efficacy and safety of flutiform® pMDI 50/5 μg in children with asthma5
Abbreviations: CI, confidence interval; FEV₁, forced expiratory volume in 1 second; FP/SAL, fluticasone/ salmeterol LS, least square; pMDI, pressurised metered-dose inhaler
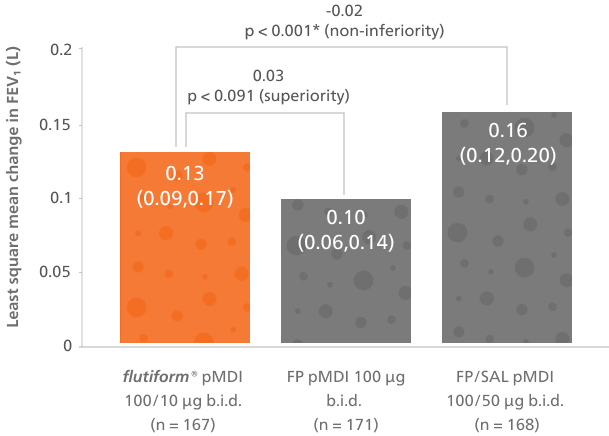
Key Outcomes:5
The change in predose FEV₁ from day 0 to day 84 demonstrated that flutiform® pMDI 50/5 μg was non-inferior to FP/SAL pMDI 50/25 μg. LS mean treatment difference was −0.031 (95.35% CI, −0.093, 0.031; p = 0.026).5
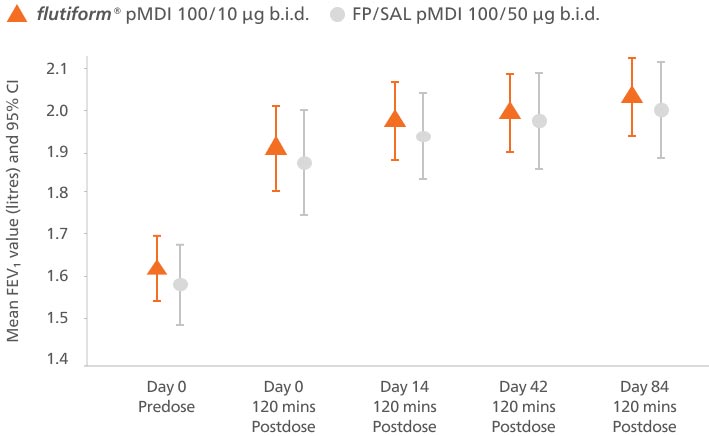
Adapted from Emeryk A, et al. 2016
Mean predose FEV₁ (with 95% CI) during the core study (per-protocol set)
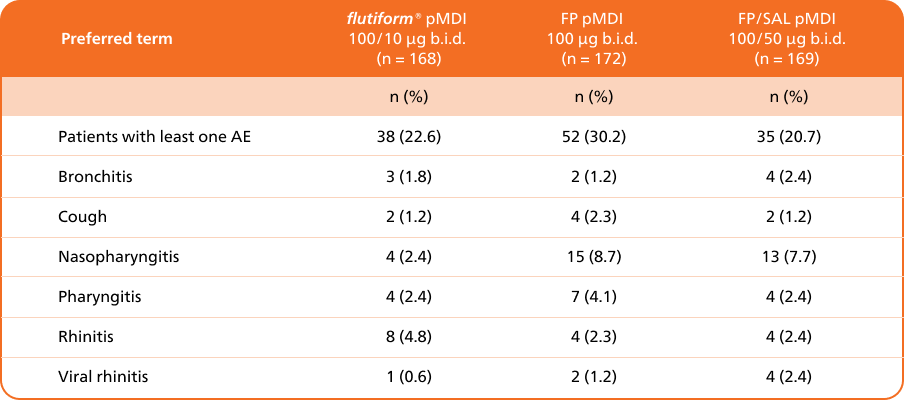
Tolerability & safety profile
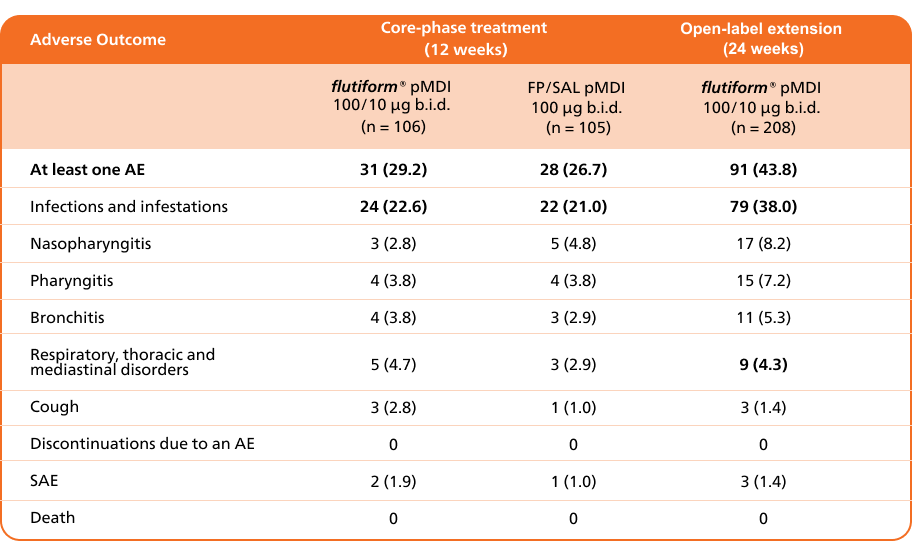
flutiform® pMDI 50/5 µg demonstrated acceptable safety and tolerability during 12 weeks of randomised therapy compared with fluticasone propionate/ salmeterol pMDI 50/25 µg and throughout the 24-week extension phase in children with asthma.5

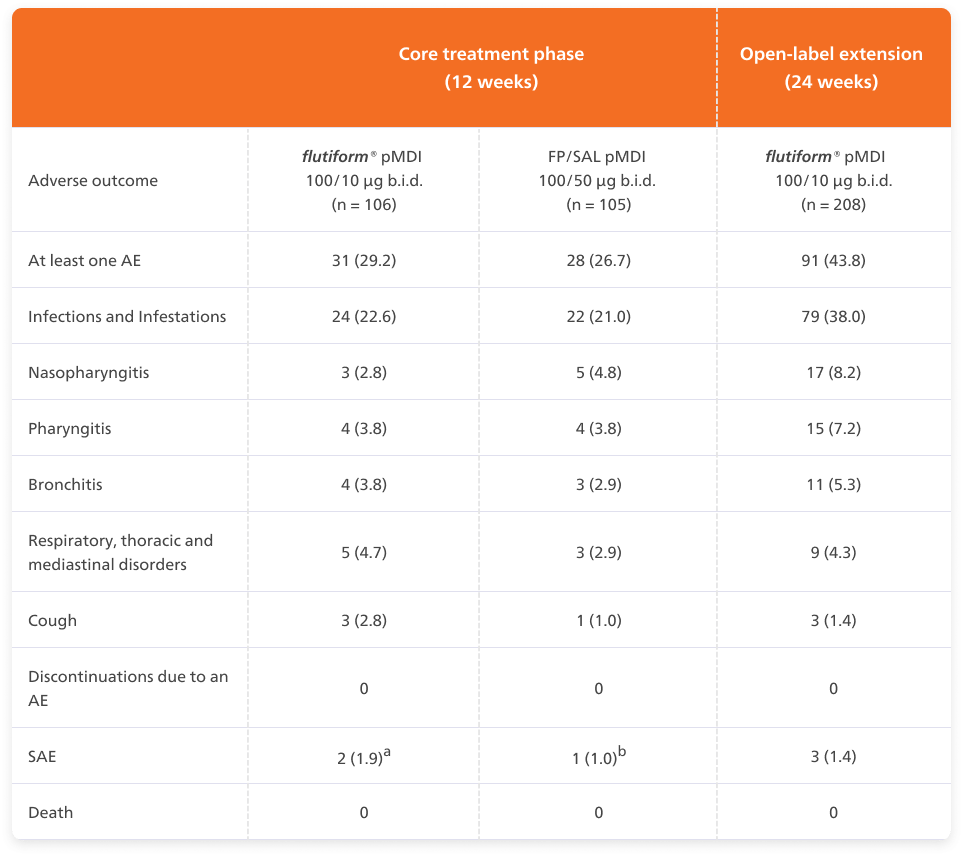
Adapted from Emeryk A, et al. 2016
a Two cases of appendicitis considered not related to study medication. b Pneumonia, considered not related to study treatment.
Abbreviations: b.i.d., twice daily; AE, adverse event; n, number of patients in a treatment group; SAE, serious adverse event
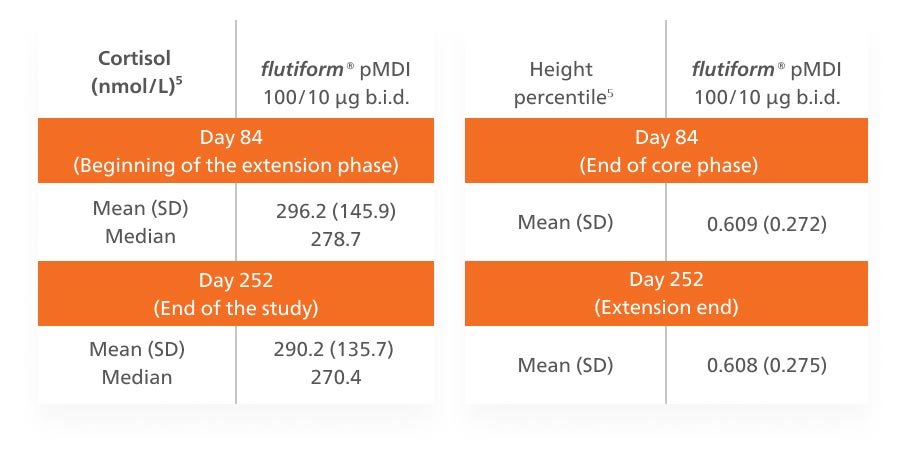
Effects of flutiform® pMDI 50/5 µg on plasma cortisol, height, and exacerbations:5
- No hypothalamic pituitary adrenal-axis suppression was observed.5
- Plasma cortisol values were within the normal range at the beginning of the extension phase and remained stable up to study end in the majority of patients.
- Mean and median plasma cortisol values remained stable over the 24 weeks.
- Mean height increased by 2.8 cm during the extension phase.5
- This change was as expected in children aged 4-12 years.
- There were no cases of severe exacerbations.5
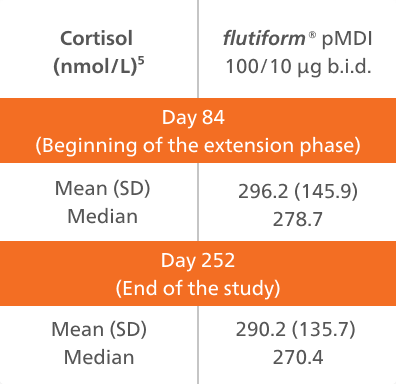
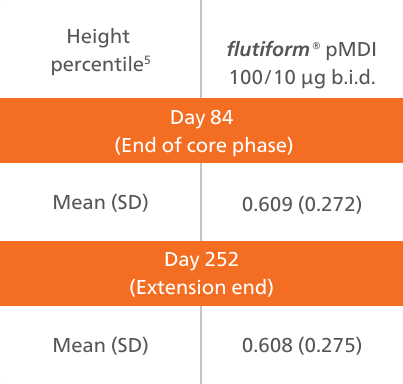
A post hoc analysis was conducted to evaluate any effect of flutiform® pMDI 50/5 μg on patient growth based on height and weight data between day 84 (extension start) and day 252 (extension end), with reference to standardised height and weight tables [National Center for Health Statistics – CDC, 2000].5
Find out more about the indication and dosing for flutiform® pMDI
Abbreviations: SD, standard deviation; pMDI, pressurised metered-dose inhaler; b.i.d., twice daily
References
- Network GA. The Global Asthma Report, Auckland, New Zealand (2018).
- Dharmage SC, et al. Front Pediatr 2019;7:246.
- flutiform® SmPC. Last updated 13 April 2022. https://www.emcpi.com/pi/26954. Accessed 5 September 2022.
- Płoszczuk A, et al. Ther Adv Respir Dis 2018.
- Emeryk A, et al. Ther Adv Respir Dis 2016;10:324-37.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (Updated 3rd May 2022). Available at: https://ginasthma.org/wp-content/uploads/2022/05/GINA-Main-Report-2022-FINAL-22-05-03-WMS.pdf Accessed 5 September 2022.

Let's connect
Join your colleagues and sign up for the latest news on the management of asthma and flutiform® pMDI straight to your inbox.
®: FLUTIFORM is the Trademark of Jagotec AG used under licence by Mundipharma.
®: The ‘lung’ logo, is a Registered Trademark of Mundipharma.