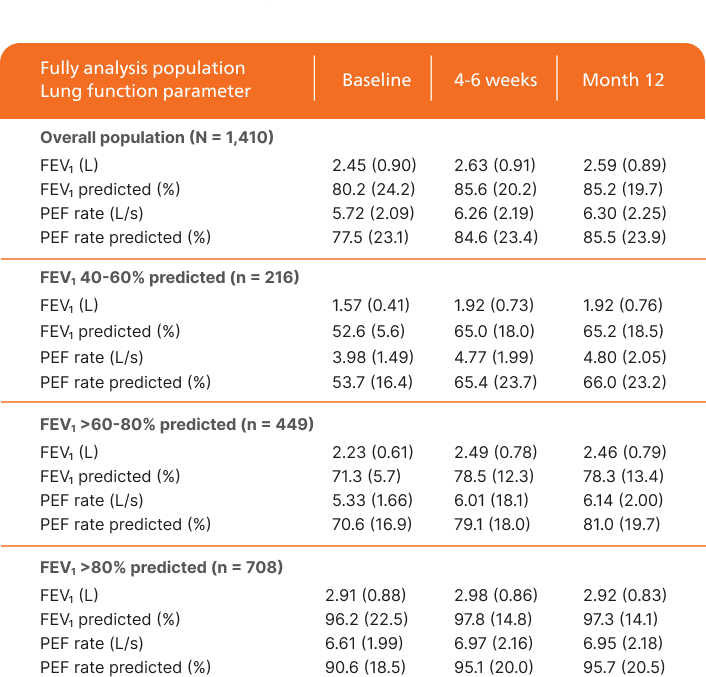
Improvement in lung function over time1
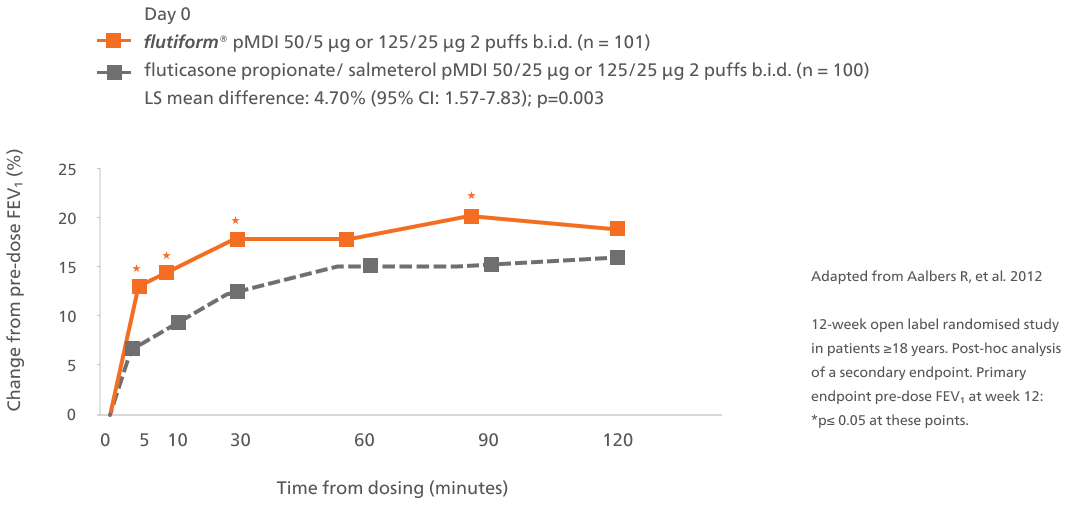
Abbreviations: b.i.d., twice daily; CI, confidence interval; LS, Least square; FEV₁, forced expiratory volume in 1 second; pMDI, pressurised metered-dose inhaler
Real-world evidence
(adolescent and adult studies)
flutiform® pMDI has demonstrated improved levels of asthma control compared to baseline (as per GINA guidelines3) over 3 and 12 months across different RWE studies enrolling more than 4,000 patients4-6
Abbreviations: GINA, Global Initiative for Asthma; RWE, real-world evidence; pMDI, pressurised metered-dose inhaler
ffLUX4
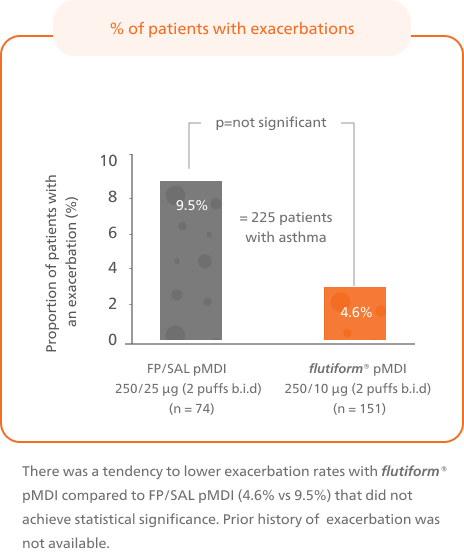
A significantly higher proportion of patients achieved controlled asthma after switching from fluticasone propionate/ salmeterol to flutiform® pMDI4
ffLUX4: A randomised pragmatic trial changing from fluticasone propionate/ salmeterol pMDI to flutiform® pMDI (12 weeks)
Key Outcomes:4
Phase 1 (Switch)
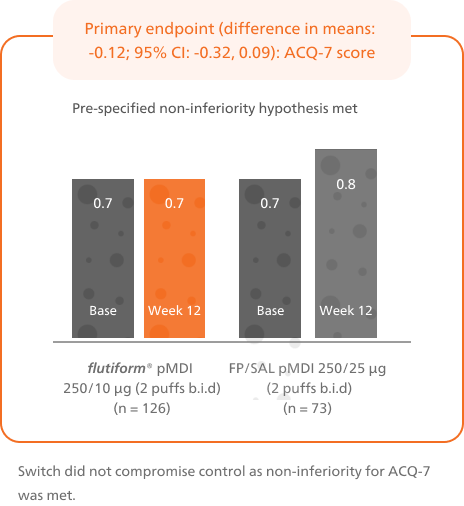
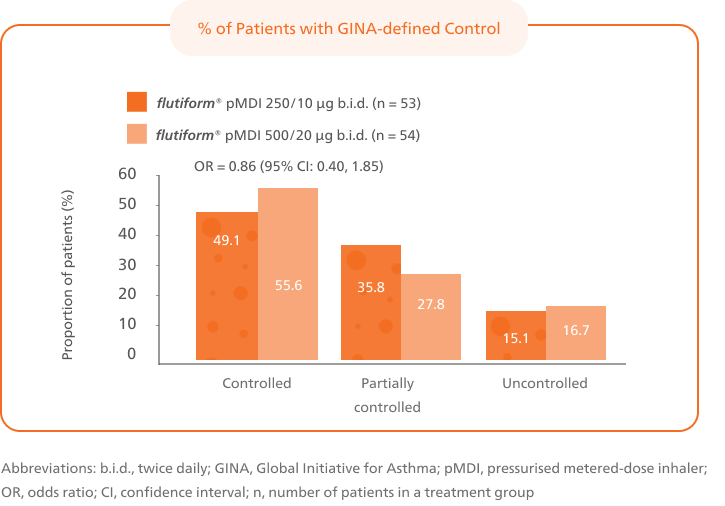
Patients switched from fluticasone propionate/ salmeterol pMDI to flutiform® pMDI had significantly higher odds of achieving asthma control as per GINA-defined criteria3 (on the basis of symptoms in the last week) at week 12.4
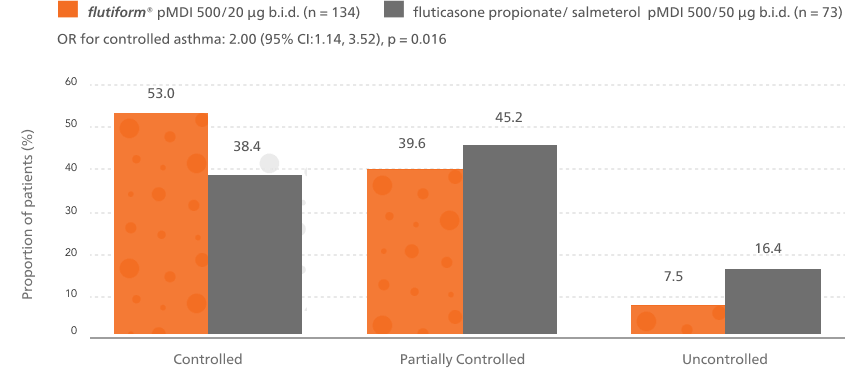
Phase 1 outcome of the study
This is based on GINA defined criteria (on the basis of symptoms in the last week).3
Abbreviations: b.i.d., twice daily; CI, confidence interval; GINA, Global Initiative for Asthma; OR, odds ratio; pMDI, pressurised metered-dose inhaler; pMDI; pressurised metered-dose inhaler
ffAIRNESS5
The proportion of patients with well-controlled asthma more than doubled after 12 months on flutiform® pMDI5
ffAIRNESS5: Real-life effectiveness of asthma treatment with a fixed-dose flutiform® pMDI
Prospective, 1-year, non-interventional, multi-centre study in Germany
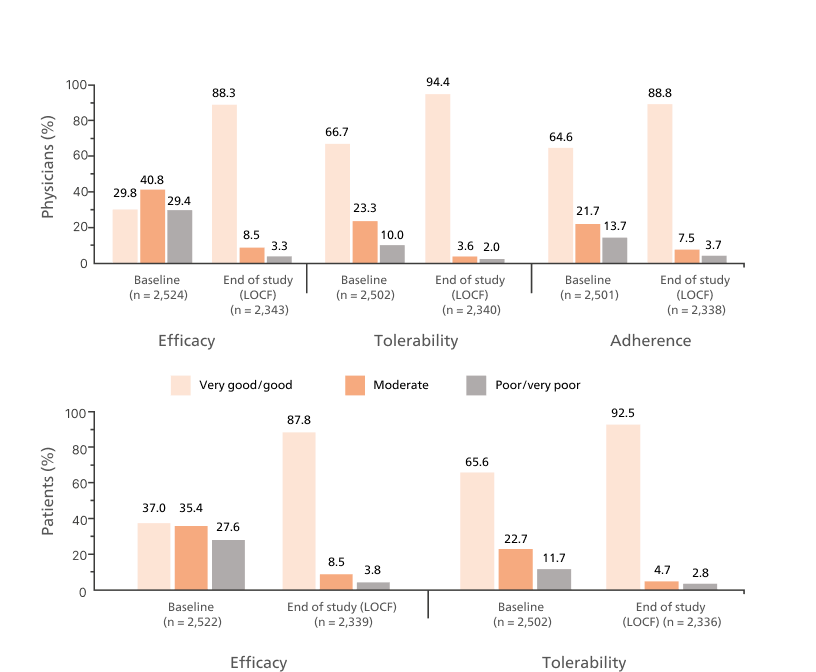
Key Outcomes:5
Asthma Control Test™ Scores
Proportion of patients with well-controlled asthma from baseline (30.9%) to 65.3% after 12 months on flutiform® pMDI.5
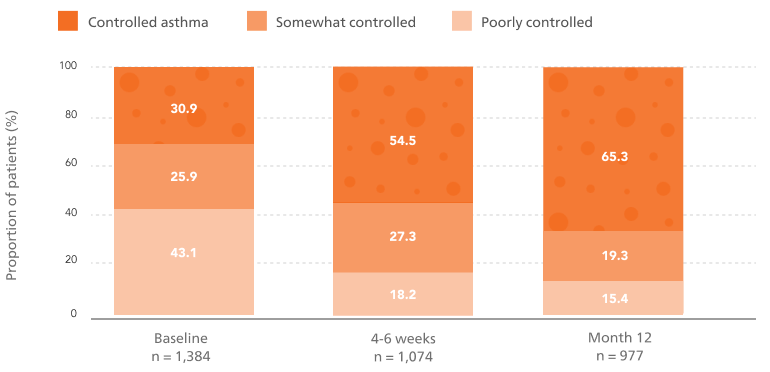
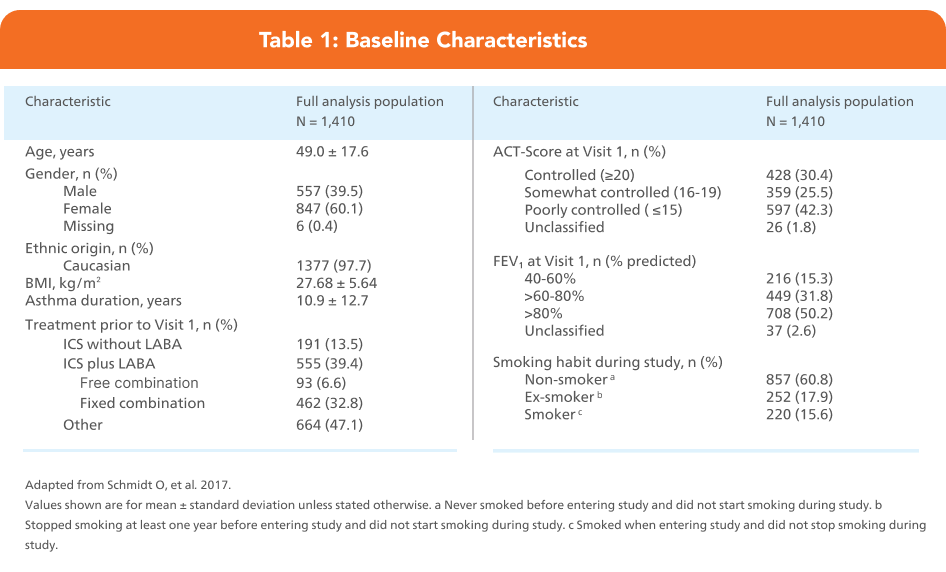
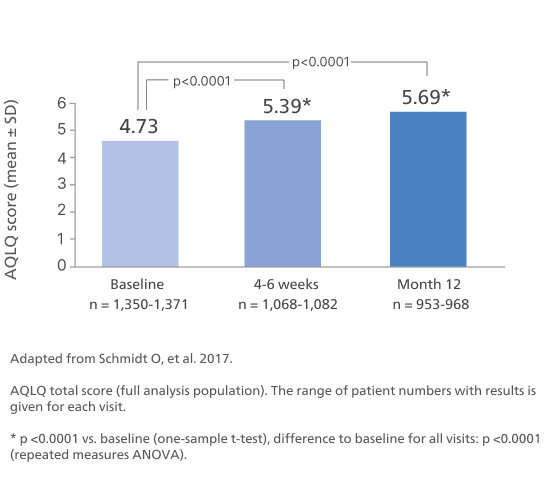
Adapted from Schmidt O, et al. 2017.
Full analysis population = 1,410
Last observation carried forward results show that 62.4% of patients achieved ACT scores ≥ 20
Abbreviation: ACT, Asthma Control Test; pMDI, pressurised metered-dose inhaler
AffIRM6
An increased proportion of patients with controlled asthma from baseline to the end of the study (12 months).6
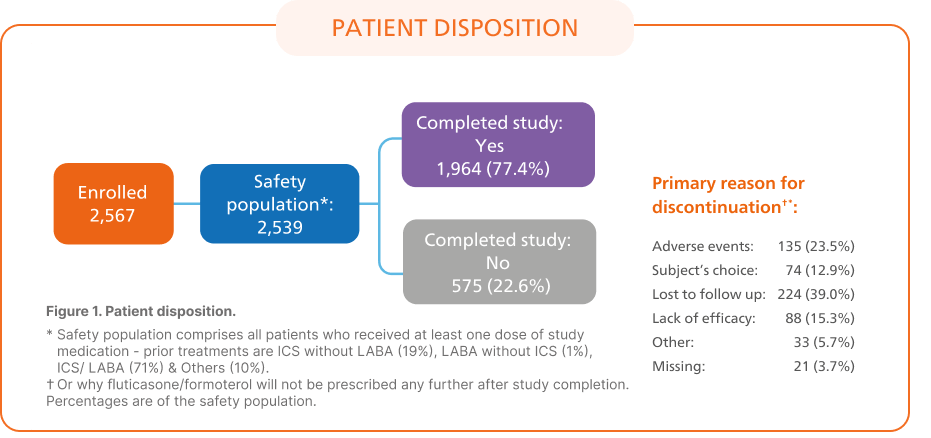
AffIRM6: Real-world study to evaluate the safety and effectiveness of flutiform® pMDI in 2,539 patients with asthma
Key Outcomes:6
Asthma Control Test™ Scores
The proportion of patients with controlled asthma increased from 29.4% at baseline to 67.4% at end of study (12 months).6
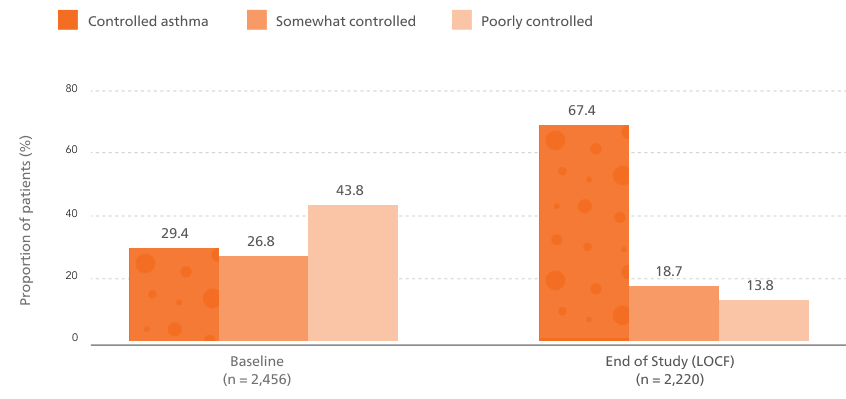
Asthma control based on ACT total score (controlled, ACT ≥ 20; somewhat controlled, ACT 16-19; poorly controlled, ACT ≤ 15)
Baseline data reflect prior treatment
Abbreviations: ACT, Asthma Control Test; LOCF, last observation carried forward up to 12 months; pMDI, pressurised metered-dose inhaler
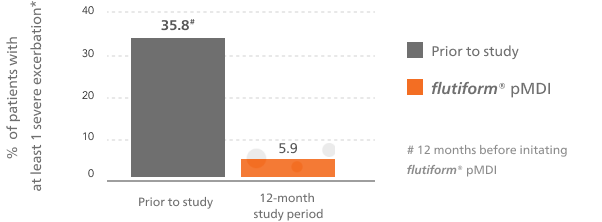
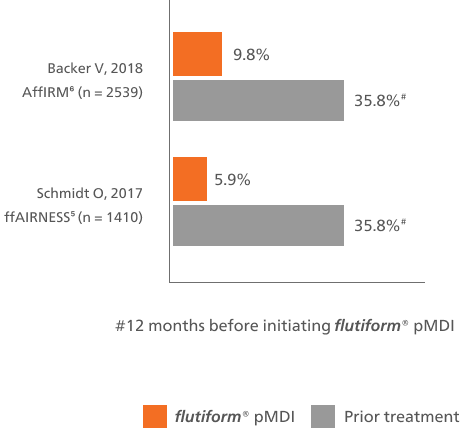
Associated with a low rate of severe asthma exacerbations5-7
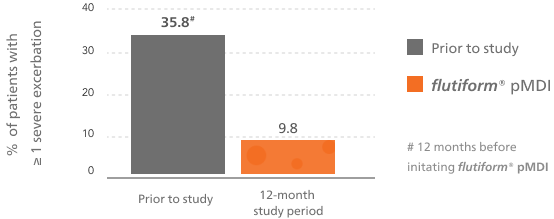
Long-term therapy with flutiform® pMDI is associated with a low rate of severe asthma exacerbations in RCTs (2.1%)7 and RWE.5,6
Randomised Controlled Trials (RCTs)7
Oral corticosteroid-requiring exacerbation rates across individual studies of ICS/LABA combinations7
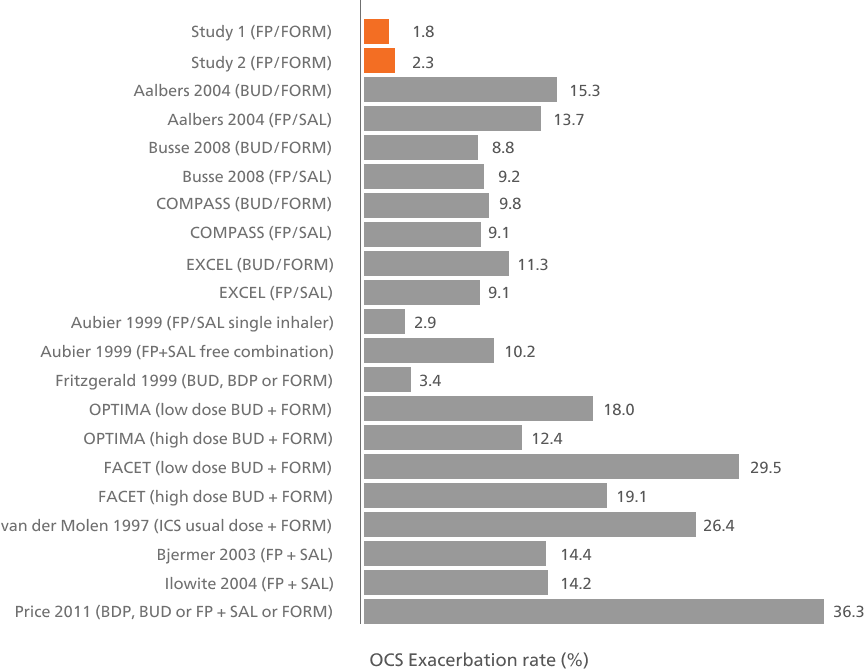
Adapted from Papi A, et al. 2016. In these studies, ICS/LABAs were only used as maintenance therapy.
Real-World Evidence (RWE)5,6
flutiform® pMDI reduced the rates of severe asthma exacerbations vs baseline treatment5,6
This bar chart shows at least one severe asthma exacerbation during the observational period of the study for flutiform® pMDI compared with the 12 months prior to the start of the study.
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting ß₂-agonist; FP, fluticasone propionate; FORM, formoterol; BUD, budesonide; SAL, salmeterol; BDP, beclomethasone dipropionate; OCS, oral corticosteroid; pMDI, pressurised metered-dose inhaler; n, number of patients in a treatment group
References
- Aalbers R, et al. Adv Ther. 2012; 29(11): 958-969.
- flutiform® SmPC. Last updated 13 April 2022. https://www.emcpi.com/pi/26954. Accessed 5 September 2022.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (Updated 3rd May 2022). Available at: https://ginasthma.org/wp-content/uploads/2022/05/GINA-Main-Report-2022-FINAL-22-05-03-WMS.pdf Accessed 5 September 2022.
- Usmani OS, et al. J Allergy Clin Immunol Prac 2017;5:1378-87.
- Schmidt O, et al. Respir Med 2017;131:166-174.
- Backer V, et al. Ther Adv Respir Dis 2018;12:1-16.
- Papi A, et al. J Aerosol Med Pulm Drug Deliv. 2016;29:346-361.
Would you like to know more?
Join your colleagues and sign up for the latest news on the management of asthma and flutiform® pMDI straight to your inbox.
Abbreviation: pMDI, pressurised metered-dose inhaler

®: FLUTIFORM is the Trademark of Jagotec AG used under licence by Mundipharma.
®: The ‘lung’ logo, is a Registered Trademark of Mundipharma.